Kainos

国别:日本Kainos
The FGF-23 ELISA Kit is a two-site enzyme-linked immunosorbent assay kit for the measurement of FGF-23 in serum. Two specific murine monoclonal antibodies bind to full-length FGF-23. One antibody is immobilized onto the microtiter plate well for capture. The other antibody is conjugated to HRP(horseradish peroxidase) for detection.
In first reaction, a sample containing FGF-23 is incubated with the immobilized antibody in a microtiter well. FGF-23 in the sample is captured with the antibody. At the end of this reaction, the well is washed to remove unbound FGF-23 and other components.
In second reaction, this immobilized FGF-23 is incubated with HRP labeled antibody to form a “sandwich”complex :
Anti FGF-23 antibody - N-terminal FGF-23 C-terminal - HRP labeled anti FGF-23 antibody
At the end of this reaction, the well is washed to remove unbound components.
In enzyme reaction, the sandwich complex immobilized on the well is incubated with a substrate solution and then measured by a spectrophotometric microtiter plate reader. The enzymatic activity of the complex bound to the well is directly proportional to the amount of FGF-23 in the sample.
A standard curve is generated by plotting the absorbance versus the each concentration of FGF-23 standard. The concentration of FGF-23 in the sample is determined from this curve.
This kit captures ADAMTS13-cleaved products using a sandwich method with the anti-GST mouse monoclonal antibody and the peroxidase-conjugated mouse monoclonal anti-N10 antibody. First, the anti-GST mouse monoclonal antibody immobilized on to the microplate reacts with the VWF73 substrate(GST-VWF73-His). The sample is then applied to the microplate, on which ADAMTS13 cleaves the VWF73 substrate. By applying the mouse monoclonal anti-N10 antibody conjugated with horseradish peroxidase(HRP conjugated antibody), the cleavage product is sandwiched between the two antibodies. Because the amount of the cleavage products depends on the ADAMTS13 activity, the amount of the enzyme-labeled antibodies that bind to the cleavage products also reflect the level of ADAMTS13 activity. Therefore, the plasma ADAMTS13 activity can be determined by colorimetrically measuring the amount of detached oxidized (colored)TMBZ using urea hydrogen peroxide(H2O2)and 3,3’,5,5’-Tetramethyl- benzidine(TMBZ)as the substrates.

Kainos
Aqua-auto Kainos UN-II Test Kit
While the Urease-GLDH method is most widely used for measuring urea nitrogen with its high sensitivity and specificity, this method suffers from possible erroneous results because of presence of ammonium that may be generated with bacterial contamination.
Kainos’ newly developed Aqua-auto Kainos UN-II Test Kit that uses isocitric acid dehydrogenase (ICDH) to dramatically improve the coping capacity to ammonium in the sample is a liquid form urea nitrogen measurement reagent kit with superior manipulability and linearity for various automatic analyzer systems.

Kainos
Aqua-auto Kainos CRE-II Test Kit
Serum creatinine level is an index of high diagnostic value that increases with impaired renal functions such as those caused by kidney failure or uremia.
Kainos' newly developed Aqua-auto Kainos CRE-II Test Kit that is based on enzymes with excellent heat stability along with proprietary technology in the added stabilizer and dye is an easy-to-use liquid form creatinine measurement reagent kit for various makes of automatic analyzer systems.

Kainos
Aqua-auto Kainos ALB Test Kit
The Bromocresol Green (BCG) method commonly used for colorimetric determination of albumin suffers from the disadvantage that it also reacts with the globulin fraction.
Kainos’ new Aqua-auto Kainos ALB Test Kit is a liquid form reagent kit based on the Bromocresol Purple (BCP) method that has a superior specificity to albumin compared to the traditional BCG method, solving the issue of different reactivities that BCP exhibits toward nonmercaptalbumin (HNA, oxidized form) and mercaptalbumin (HMA, reduced form).

Kainos
POCT refers to tests that are performed in close proximity to patients including those tests performed in hospital ICUs, emergency treatment rooms, hospital wards or clinical practices including offices of practicing doctors as well as patient-performed tests such as the blood glucose monitoring tests. Because they can quickly provide results which doctors can immediately use to choose appropriate remedies, they are now widely used in various situations including diagnosis of influenza viruses and pregnancy.
“Swiftgene Norovirus GI/GIIKainos” for detecting noroviruses
This product qualitatively detects norovirus genes extracted from fecal or other samples through a NASBA nucleic acid amplification technology combined with a nucleic acid chromatography.

Kainos
“StatmarkTM FLUstick-N” for detecting Type A and Type B influenza virus antigens
This product detects the Type A and/or Type B antigens to influenza viruses in nasal wipe or discharge samples using an immunochromatographic technique.

Kainos
|
Qty
|
Package Size (LxWxH)
|
Approx. Weight
|
|
1 Kit
|
28x37x29 cm
|
6.0 kg
|
|
2 Kits
|
28x37x29 cm
|
6.5 kg
|
|
3 Kits
|
35x42x30 cm
|
7.5 kg
|
|
4 Kits
|
35x42x30 cm
|
8.0 kg
|
|
5 Kits
|
35x42x30 cm
|
9.0 kg
|
|
10 Kits
|
37x66x32 cm
|
15.0 kg
|
PERFORMANCE
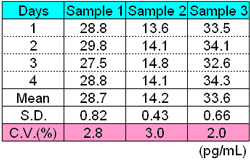
Callibration curve
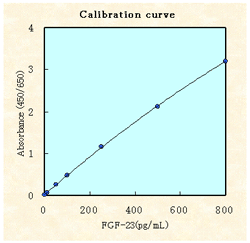
Linearity (Human serum)
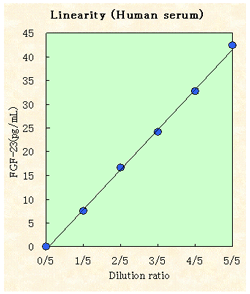
Sensitivity
(n=8, mean±2 S.D.
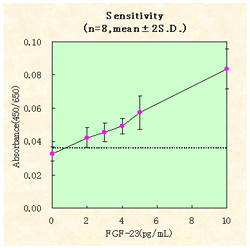
INTER-ASSAY
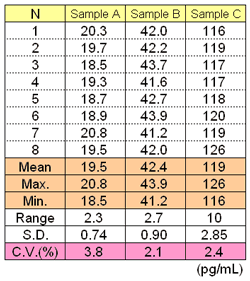
INTRA-ASSAY